Abstract
Diffuse large B-cell lymphoma (DLBCL) accounts for 30-40% of adult non-Hodgkin lymphomas. Most DLBCL patients achieve long-term remission after treatment, but a third relapse after conventional Rituximab (R)-based chemotherapy regimens, such as CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) (Siegel et al, 2012).
Cancer cells are exposed to chronic replication stress, which impedes the duplication of their genome and induces mitotic catastrophe (Shaheen et al, 2011). Functional DNA repair pathways are therefore important for the survival of cancer cells. This dependence can be exploited therapeutically to hamper repair of the intrinsic DNA damage occurring during replication or to exacerbate DNA damage induced by chemotherapy (Shaheen et al, Blood 2011). Furthermore, high-risk DLBCL patients overexpress genes potentially involved in resistance to CHOP-based regimens, such as genes of the nucleotide excision repair (NER) pathway (Bret et al, Oncotarget 2012, Cell Cycle 2013).
We recently developed GEP-based DNA repair scores that allow to identify high-risk DLBCL patients that could benefit from treatment with DNA repair inhibitors (Bret at al, BJH 2015).
DLBCL treatments include cyclophosphamide, a nitrogen mustard derivate that induces interstrand crosslinks (ICLs), and doxorubicin, a DNA topoisomerase inhibitor that induces DNA double-strand breaks, DNA adducts and formaldehyde-dependent ICL formation. Inhibiting DNA repair is a promising strategy to improve the efficacy of genotoxic drugs and overcome drug resistance. Our data support the view that inhibitors of DNA damage signaling and DNA repair have potential therapeutic interest in DLBCL.
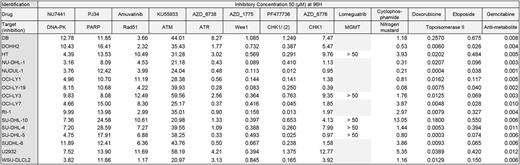
We characterized the drug-response of 16 DLBCL cell lines to 9 DNA repair inhibitors including PJ34 (PARP inhibitor), NU7441 (DNAPK inhibitor), KU55933 (ATM inhibitor), PF477736 (CHK1/2 inhibitor), AZD6738 (ATR inhibitor), MK8776 (CHK1 inhibitor), AZD1775 (Wee1 inhibitor), MP-470 (Rad51 inhibitor), Lomeguatrib (MGMT) and genotoxic agents used in DLBCL treatment (Cyclophosphamide, Gemcitabine, Doxorubicin and Etoposide) (Table 1). All drugs induced significant apoptosis (Annexin V staining and PARP cleavage) and significant inhibition of proliferation (BrdU incorporation) in the different DLBCL cell lines tested (P <0.05). CHK1/2 inhibitor, Wee1 inhibitor, Cyclophosphamide, Gemcitabine and Doxorubicin induced DNA damages monitored by H2AX phosphorylation. Correlating drug response of each compounds with our GEP-based DNA repair scores (Bret et al, BJH 2015), we identified a significant correlation between FANC score and response to ATR inhibitor (AZD6738) and HRR score/BER score and response to Etoposide (P<0.05). High-risk DLBCL patients identified with GEP-based FANC, HRR and BER scores may benefit from treatment by ATR inhibitors or Etoposide respectively.
Since DNA repair pathways play a role in drug resistance, we sought to identify new synthetic lethal and synergistic combinations associating IC20 of DNA repair targeted treatments with conventional genotoxic agents in DLBCL. Applying a standard threshold of 2 SDs below the IC50 of the genotoxic agent alone, a total of 3 synthetic lethal combinations have been identified including cyclophosphamide with CHK1/2 inhibitor (PF477736) (IC50 reduced from 2.63 µM to 1.20 µM), cyclophosphamide and ATR inhibitor (AZD6738) (IC50 reduced from 2.63 µM to 0.74 µM) and doxorubicin with DNAPK inhibitor (NU7441) (IC50 reduced from 57 nM to 14 nM).
Furthermore we identified new potent synergistic combinations (combination index < 1) including CHK1/2 inhibitor (PF477736) with etoposide (IC50 reduced from 560 nM to 437 nM), ATR inhibitor (AZD6738) (IC50 reduced from 560 nM to 364 nM) with etoposide and ATM inhibitor (KU55933) with etoposide (IC50 reduced from 560 nM to 269 nM).
Despite overall improvements in the treatment of DLBCL, including the use of rituximab, approximately one-third of patients fail to achieve complete remission or experience relapse. This remains a major cause of morbidity and mortality. The DNA repair scores could be useful to identify high-risk DLBCL patients and define the best synthetic lethal approach combining DNA repair inhibitors with conventional chemotherapy. These results open new perspectives to improve the treatment of DLBCL patients and provide new strategies to overcome drug resistance.
Cartron: Sanofi, BMS, Jansen, celgene, Roche, Gilead: Equity Ownership; Celgene: Consultancy, Employment; Roche: Consultancy, Equity Ownership, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal